
Here we summarize the reactions that occur in the ETC to produce energy, but for a more detailed review, refer to Zhao et al.

However, the molecules derived from these processes are used in the tricarboxylic acid (TCA) cycle to generate substrates that enter the ETC for oxidative phosphorylation. The processes for the catabolism of glucose (via glycolysis and subsequent pyruvate oxidation), fatty acids (via fatty acid β-oxidation), and amino acids (via oxidative deamination and transamination) are reviewed in detail elsewhere. Further, we will compare current methodologies to measure bioenergetic function and mitochondrial ROS production.Ĭellular metabolism comprises the utilization of carbohydrates, fats, and proteins, to synthesize energy. In this review, we will provide an overview of the function of the ETC, focusing on oxidative phosphorylation and its relationship to ROS production. ĭespite this variation between cell types, mitochondrial ATP generation and ROS production are intimately linked through function of the electron transport chain (ETC), and thus efficient measurement of ETC function can provide insight into mechanisms of physiology and disease pathogenesis. In contrast, endothelial cells rely more heavily on glycolysis than mitochondria for ATP, but mitochondrial ROS production is essential for endothelial homeostatic signaling. For example, cardiomyocytes rely on mitochondria to supply >95% of the energy required for their function. Notably, the importance of each of these functions varies by cell type. Since then, it has become apparent that mitochondria are highly dynamic organelles that contribute to cellular homeostasis not only through maintaining adenosine triphosphate (ATP) levels, but also through the generation of low levels of ROS for cell signaling, and that dysfunction in either of these processes can propagate pathology. Less than a decade later came the first reports that the organelle generated reactive oxygen species (ROS) as a byproduct of cellular respiration. They absorb photons with high efficiency so that whenever a pigment in the photosynthetic reaction center absorbs a photon, an electron from the pigment is excited and transferred to another molecule almost instantaneously.In 1957, Peter Siekevitz branded the mitochondrion the “powerhouse” of the cell. The two photosystems performing all of this magic are protein complexes that are similar in structure and means of operation.
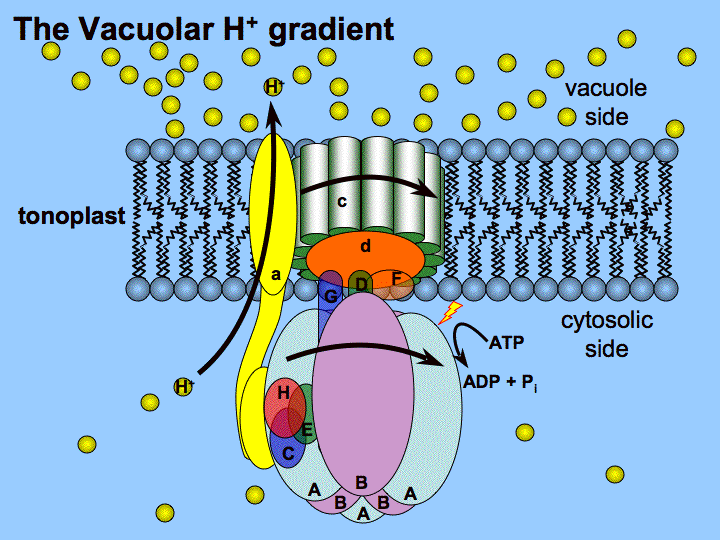
Energy for the entire process came from four photons of light. The electrons have made their way from water to NADPH via carriers in the thylakoid membrane and their movement has released sufficient energy to make ATP. At this point, the light cycle is complete - water has been oxidized, ATP has been created, and NADPH has been made. Note that reduction of NADP+ to NADPH requires two electrons and one proton, so the four electrons and two protons from oxidation of water will result in production of two molecules of NADPH. Ferredoxin then passes the electron off to the last protein in the system known as Ferredoxin:NADP+ oxidoreductase, which gives the electron and a proton to NADP+, creating NADPH. Meanwhile, the excited electron from PS I passes through an iron-sulfur protein, which gives the electron to ferredoxin (another iron sulfur protein). PS I gains a positive charge as a result of the loss of an excited electron and pulls the electron in plastocyanin away from it. With absorption of a photon of light by PS I, a process begins, that is similar to the process in PS II. Cb6f drops the electron off at plastocyanin, which holds it until the next excitation process begins with absorption of another photon of light at 700 nm by PS I. ATP synthase makes ATP from the proton gradient created in this way. PQH2 passes these to the Cytochrome b6f complex (Cb6f) which uses passage of electrons through it to pump protons into the thylakoid space.


 0 kommentar(er)
0 kommentar(er)
